Protein Isolation & Purification
Protein purification involves separating proteins based on their characteristics such as size, charge, or binding ability. The main steps include:
1. Centrifugation
The sample is spun at high speed to separate components by density. Heavy cell debris forms a pellet, while the protein-containing supernatant is collected. Often used as the first step.
2. Precipitation
Precipitation changes protein solubility by using salts (e.g., ammonium sulfate), pH shifts, or organic solvents to make proteins aggregate and fall out of solution. Useful for concentrating and roughly separating proteins.
3. Chromatography
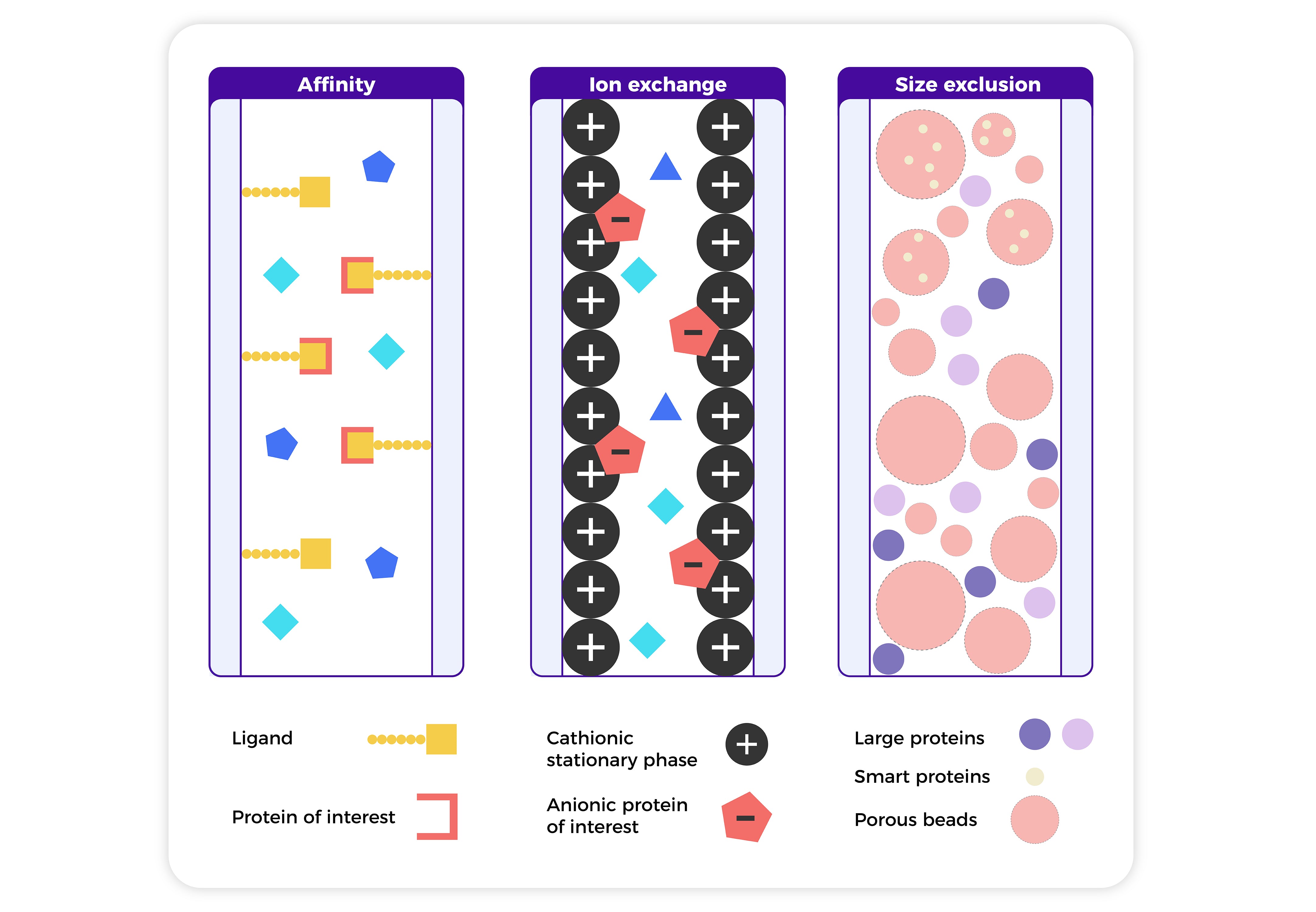
3.1 Affinity Chromatography
Uses specific binding between a protein and a ligand (e.g., His-tag and Ni column) to isolate the target protein with high selectivity.
3.2 Ion Exchange Chromatography
Separates proteins by charge. Proteins bind to charged resins and are eluted by changing pH or salt concentration.
3.3 Size Exclusion Chromatography (SEC)
Separates proteins by size. Large proteins flow out first, while smaller ones enter porous beads and elute later. Often used in the final polishing step.
The following figure and table summarize the common chromatography setups and compare various purification techniques in terms of their strengths and limitations.
Fig1. A schematic representation of columns used in typical chromatography techniques for protein purification.
Table 1: Summary of protein purification techniques with their strengths, limitations and the most suitable samples.



